The synthesis and characterization of benzotriazole-based cationic surfactants and the evaluation of their corrosion inhibition efficiency on copper in seawater
Abstract
This study aims at preparing three cationic surfactants based on benzotriazole and evaluating their efficiencies as corrosion inhibitors for copper electrodes in seawater using different electrochemical techniques (potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and atomic force microscopy (AFM)). FTIR and 1H NMR spectroscopic techniques confirmed the chemical structures of the as-prepared cationic compounds. The inhibition efficiency increased with the increase the concentration of the as-prepared compounds in the solution. The curves of the potentiodynamic polarization and the plots of EIS techniques show that the performance of all investigated compounds as mixed type. The standard free energy 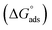 values imply that the three as-prepared compounds show physicochemical adsorption and obey the Langmuir adsorption model. AFM technique observed the reduction in the surface roughness due to the protective film formed on the copper surface. Finally, computational calculations show a great correlation with the experimental results due to the electron-donating effect.
values imply that the three as-prepared compounds show physicochemical adsorption and obey the Langmuir adsorption model. AFM technique observed the reduction in the surface roughness due to the protective film formed on the copper surface. Finally, computational calculations show a great correlation with the experimental results due to the electron-donating effect.



 Please wait while we load your content...
Please wait while we load your content...