The nickel-catalyzed C3-acylation of 2H-indazoles with aldehydes†
Abstract
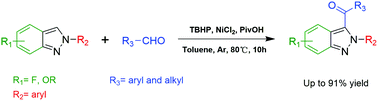
A direct coupling of 2H-indazoles’ C3 position and acyl groups has been achieved to produce 3-acyl-2H-indazoles. The Ni(II)-catalyzed acylation might proceed through a radical pathway for the reaction of 2H-indazoles with either aryl or alkyl aldehydes in the presence of the free radical initiator TBHP and additive PivOH. This method provided a superior approach to fulfil the direct C3-acylation of 2H-indazoles with yields up to 91%. And various substituted 2H-indazoles were well tolerated with this method, enriching the diversity of 2H-indazole derivatives. In comparison with previously reported approaches for the C3-acylation of 2H-indazoles, the developed reaction represents a more convenient and economical method directly using aldehydes as the acylation agents.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...