Catalytic asymmetric synthesis of 3-aryl phthalides enabled by arylation–lactonization of 2-formylbenzoates†
Abstract
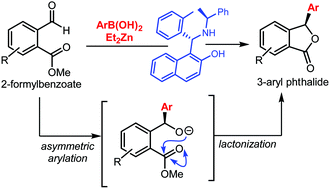
The catalytic asymmetric synthesis of 3-aryl phthalides is reported through a sequential asymmetric arylation–lactonization reaction. The reaction is enabled by a boron–zinc exchange to generate reactive arylating agents, which react with 2-formylbenzoates in the presence of a chiral amino naphthol ligand. The enantiodetermining step is the arylation of the aldehyde, which then undergoes a lactonization event to yield the corresponding phthalides in good yields and enantioselectivities.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...