Pyrrolocytosine–pyrene conjugates as fluorescent and CD probes for the fine sensing of ds-polynucleotide secondary structure and specific recognition of poly G†
Abstract
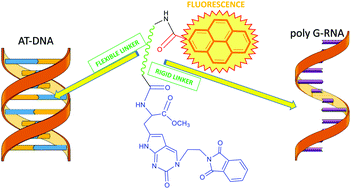
Three novel pyrene–pyrrolocytosine (PyrrC) conjugates efficiently bind to ds-DNA/RNA grooves. The compounds exhibit DNA/RNA secondary structure-dependent fluorimetric selectivity. The most pronounced differentiation was observed between poly(dAdT)2 and poly A–poly U. Moreover, the most flexible conjugate showed the specific fluorescence band at 470 nm with poly(dAdT)2. The different induced circular dichroism (I)CD response of rigid and flexible pyrene–pyrrC conjugates upon A–T(U) binding revealed the fine structural tuning of the pyrene position within the DNA/RNA binding site. The novel conjugates have shown unmatched specific fluorimetric recognition of poly G to date, with respect to other ss-RNA, attributed to the synergistic effect of pyrene intercalation combined with H-bonding recognition of the complementary base pair. The most prominent response for poly G was observed for the most rigid conjugate, having a conveniently pre-organized conformation. All the conjugates efficiently entered into the human cells, showing no cytotoxicity, thus giving rise to promising lead compounds for the further development of cellular or biochemical spectrophotometric probes.


 Please wait while we load your content...
Please wait while we load your content...