A palladium-catalyzed three-component cascade S-transfer reaction in ionic liquids†
Abstract
A palladium-catalyzed three component cascade S-transfer reaction of acetylinic oximes with aryl halides using readily available Na2S2O3 as an odorless sulfenylation reagent under aerobic conditions in ionic liquids was described. The present protocol features environmental friendliness, good functional group compatibility, odorless sulfenylation reagents, without any ligand or additive, and excellent atom and step economy. Remarkably, this cascade procedure will be bringing about further late-stage modification for the construction of structurally complex isoxazole scaffolds in synthetic and pharmaceutical chemistry.
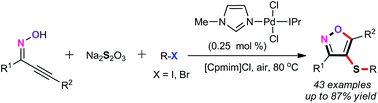


 Please wait while we load your content...
Please wait while we load your content...