Solar-driven biocatalytic C-hydroxylation through direct transfer of photoinduced electrons†
Abstract
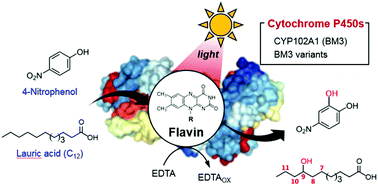
Despite the immense potential of P450s, the dependence on the nicotinamide cofactor (NADPH) and NADPH-P450 reductase (CPR) limits their employment in the chemical industry. Here, we present a visible light-driven platform for biocatalytic C-hydroxylation reactions using natural flavin molecules, especially flavin mononucleotide, as a photosensitizer. By employing visible light as a source of energy instead of the nicotinamide cofactor, the bacterial CYP102A1 heme domain was successfully applied for photobiocatalytic C-hydroxylation of 4-nitrophenol and lauric acid – in the absence of NADPH and CPR. We present a proof of concept that the photoactivation of flavins is productively coupled with the direct transfer of photoinduced electrons to the P450 heme iron, achieving photobiocatalytic C-hydroxylation reactions.


 Please wait while we load your content...
Please wait while we load your content...