The synergy of different solid-state techniques to elucidate the supramolecular assembly of two 1H-benzotriazole polymorphs†
Abstract
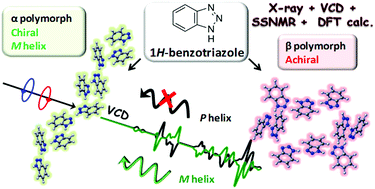
1H-Benzotriazole crystallizes as two different polymorphs, namely 4aα and 4aβ. One polymorph is chiral and it resolves spontaneously as conglomerates. The other polymorph crystallizes in a centrosymmetric space group and it is therefore achiral. In both polymorphs supramolecular structures are formed starting from achiral monomers. An analysis of these two polymorphs of 1H-benzotriazole has been carried out by a complete strategy involving different solid-state experimental techniques and quantum chemical calculations (DFT, Density Functional Theory). In particular, X-ray crystallography, NMR spectroscopy and vibrational spectroscopy techniques (FarIR, IR and Raman) that are not sensitive to chirality have been used to characterize the two polymorphs structurally. Vibrational spectroscopy (VCD, Vibrational Circular Dichroism) that is sensitive to chirality was employed to determine the absolute configuration (M or P helices) of the chiral supramolecular structure of 4aα.


 Please wait while we load your content...
Please wait while we load your content...