Probing the limits of Q-tag bioconjugation of antibodies†
Abstract
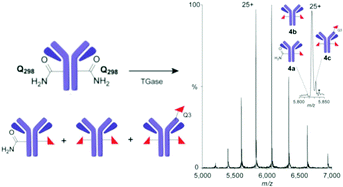
Site-selective labelling of antibodies (Abs) can circumvent problems from heterogeneity of conventional conjugation. Here, we evaluate the industrially-applied chemoenzymatic ‘Q-tag’ strategy based on transglutaminase-mediated (TGase) amide-bond formation in the generation of 89Zr-radiolabelled antibody conjugates. We show that, despite previously suggested high regioselectivity of TGases, in the anti-Her2 Ab Herceptin™ more precise native MS indicates only 70–80% functionalization at the target site (Q298H), in competition with modification at other sites, such as Q3H critically close to the CDR1 region.


 Please wait while we load your content...
Please wait while we load your content...