Regioselective cycloaddition of potassium alkynyltrifluoroborates with 3-azetidinones and 3-oxetanone by nickel-catalysed C–C bond activation†
Abstract
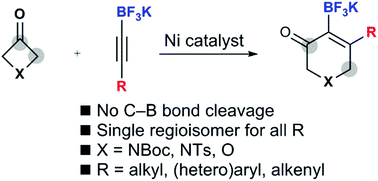
In the presence of a nickel catalyst, the intermolecular (4+2) cycloaddition of potassium alkynyltrifluoroborates with 3-azetidinones and 3-oxetanone leads to the formation of borylated dihydropyridinones and dihydropyranones without unwanted carbon–boron bond cleavage. The regioselectivity is influenced only by the trifluoroborate group, and only one regioisomer is obtained, whether the other alkyne substituent is an alkyl, vinyl, or (hetero)aryl group.


 Please wait while we load your content...
Please wait while we load your content...