Case examples of an evaluation of the human relevance of the pyrethroids/pyrethrins-induced liver tumours in rodents based on the mode of action†
Abstract
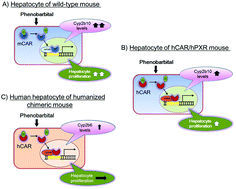
Rodent carcinogenicity studies are useful for screening for human carcinogens but they are not perfect. Some modes of action (MOAs) lead to cancers in both experimental rodents and humans, but others that lead to cancers in rodents do not do so in humans. Therefore, analysing the MOAs by which chemicals produce tumours in rodents and determining the relevance of such tumour data for human risk are critical. Recently, experimental data were obtained as case examples of an evaluation of the human relevance of pyrethroid (metofluthrin and momfluorothrin)- and pyrethrins-induced liver tumours in rats based on MOA. The MOA analysis, based on the International Programme on Chemical Safety (IPCS) framework, concluded that experimental data strongly support that the postulated MOA for metofluthrin-, momfluorothrin- and pyrethrins-produced rat hepatocellular tumours is mediated by constitutive androstane receptor (CAR) activation. Since metofluthrin and momfluorothrin are close structural analogues, reproducible outcomes for both chemicals provide confidence in the MOA findings. Furthermore, cultured human hepatocyte studies and humanized chimeric mouse liver studies demonstrated species difference between human hepatocytes (refractory to the mitogenic effects of these compounds) and rat hepatocytes (sensitive to their mitogenic effects). These data strongly support the hypothesis that the CAR-mediated MOA for liver tumorigenesis is of low carcinogenic risk for humans. In this research, in addition to cultured human hepatocyte studies, the usefulness of the humanized chimeric liver mouse models was clearly demonstrated. These data substantially influenced decisions in regulatory toxicology. In this review I comprehensively discuss the human relevance of the CAR-mediated MOA for rodent liver tumorigenesis based on published information, including our recent molecular research on CAR-mediated MOA.
- This article is part of the themed collections: Recent Review Articles and Advances in Experimental and Regulatory Toxicology


 Please wait while we load your content...
Please wait while we load your content...