The competition between mechanical stability and charge carrier mobility in MA-based hybrid perovskites: insight from DFT†
Abstract
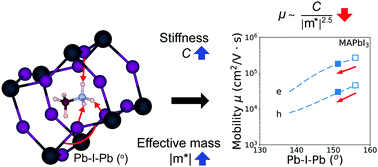
Hybrid organic–inorganic perovskites and their inorganic analogues, such as MAPbI3 (MA = methylammonium, CH3NH3) and CsPbI3, are currently under intense investigation due to their high-power conversion efficiencies and low cost for solar cell applications. Herein, we investigate the effect of methylammonium and the inorganic A-cations on the elastic and related transport properties of halide perovskites using van der Waals (vdW) corrected density functional theory (DFT) calculations. For inorganic halide perovskites we find that the bonding within the inorganic framework is mainly responsible for their elastic behavior. However, our DFT calculations show that when a MA cation is substituted into the structure the combined effects of stericity (conformation) and hydrogen-framework interactions improve the material's resistance to deformation. For example, the orientationally-averaged Young's modulus of orthorhombic MAPbI3 increases by about 19% compared to the equivalent inorganic series of structures. We also find that, within the carrier-acoustic phonon scattering regime, the electron and hole carrier mobilities of hybrid halide perovskites are lowered by the hydrogen-bonding-induced tilting of the inorganic octahedra. Taken together, these results can help guide the optimization of the mechanical and transport properties of perovskite-based solar cell materials.


 Please wait while we load your content...
Please wait while we load your content...