Hydrogen-bonded azaphenacene: a strategy for the organization of π-conjugated materials†
Abstract
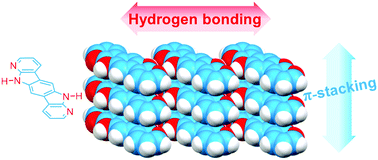
A centrosymmetric fused polyheteroaromatic system, namely 7-azaindolo[2,3-h]α-carboline, was synthesized. The pentacyclic structure includes rationally located hydrogen bond donor and hydrogen bond acceptor sites to induce its self-assembly. The molecular units associate forming an extended monodimensional arrangement that further self-organizes through π-stacking. The optical, electrochemical, X-ray diffraction, computational and electronic characterization in organic field effect transistors proves the utility of hydrogen bond-directed self-assembly as a strategy to enhance edge-to-edge interactions and orbital overlap that contribute to the charge transport properties in π-conjugated systems.


 Please wait while we load your content...
Please wait while we load your content...