The conversion mechanism of amorphous silicon to stoichiometric WS2
Abstract
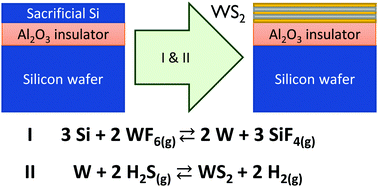
The deposition of ultra-thin tungsten films and their related 2D chalcogen compounds on large area dielectric substrates by gas phase reactions is challenging. The lack of nucleation sites complicates the adsorption of W-related precursors and subsequent sulfurization usually requires high temperatures. We propose here a technique in which a thin solid amorphous silicon film is used as reductant for the gas phase precursor WF6 leading to the conversion to metallic W. The selectivity of the W conversion towards the underlying dielectric surfaces is demonstrated. The role of the Si surface preparation, the conversion temperature, and Si thickness on the formation process is investigated. Further, the in situ conversion of the metallic tungsten into thin stoichiometric WS2 is achieved by a cyclic approach based on WF6 and H2S pulses at the moderate temperature of 450 °C, which is much lower than usual oxide sulfurization processes.


 Please wait while we load your content...
Please wait while we load your content...