A first-principles study on the adsorption of small molecules on antimonene: oxidation tendency and stability
Abstract
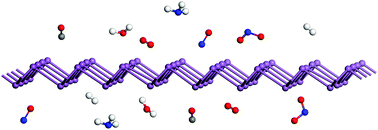
Antimonene, a new group-VA 2D semiconducting material beyond phosphorene, has been recently synthesized through various approaches and shown to exhibit good structural integrity under ambient conditions and various interesting properties. In this study, we performed systematical first-principles investigations on the interactions of antimonene with the small molecules CO, NO, NO2, H2O, O2, NH3, and H2. It was found that NO, NO2, H2O, O2, and NH3 served as charge acceptors, whereas CO showed a negligible charge transfer. H2 acted as a charge donor to antimonene, and the amount of charge transfer was ten times that of H2 on phosphorene. The interaction of the O2 molecule with antimonene is much stronger than that with phosphorene. Surprisingly, the kinetic barrier for the splitting of O2 molecule on antimonene is low (∼0.40 eV), suggesting that pristine antimonene may undergo oxidation under ambient conditions, especially at elevated temperatures. Fortunately, the acceptor role of H2O on antimonene, contrary to its donor role on phosphorene, helps to suppress further structural degradation of the oxidized antimonene by preventing proton transfer between water molecules and oxygen species to form acids. Upon comparing antimonene with phosphorene and InSe, we suspected that the acceptor role of water may be a necessary condition for good environmental stability of these 2D layers to avoid structural decomposition. Although the surface oxidation layer may serve as an effective passivation layer, preventing further degradation of the underlying layers, our findings show that the antimonene layers still need to be separated or properly protected by other noncovalent functionalization from oxygen or other environmental molecules. The present study reveals interesting insights into the environmental effects of physisorbed small molecules on the oxidation tendency and stability of antimonene that may be important for its growth, storage, and applications.


 Please wait while we load your content...
Please wait while we load your content...