Abstract
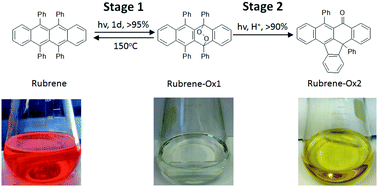
The rapid spontaneous photo-oxidation of rubrene to form endo-peroxide, rubrene-Ox1, was monitored via1H NMR and UV-vis spectroscopy. The reaction is thermally reversible, which restores high mobility devices in both the crystalline thin film and single crystal. Prolonged stirring in chlorinated solvents yields a secondary, irreversible product, rubrene-Ox2, which has lost phenol, as confirmed by single crystal analysis. An acid-catalyzed rearrangement of the endo-peroxide to form rubrene-Ox2 was identified here with Density Functional Theory (DFT). Implications of the nature of these processes for the preparation of organic transistors are described.
- This article is part of the themed collection: Celebrating 50 years of Professor Fred Wudl’s contributions to the field of organic semiconductors


 Please wait while we load your content...
Please wait while we load your content...