Abstract
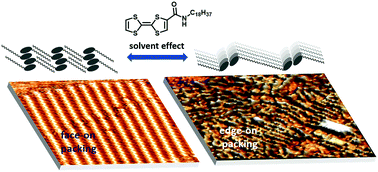
Tetrathiafulvalene (TTF) is one of the most widely used building blocks for organic conductors and redox-active materials. The ability to control the supramolecular structure of these materials, particularly at interfaces, is critical for application in devices. In this work, we show how the structure of N-alkylated tetrathiafulvalenecarboxyamide TTFAm18 films on graphite can be tuned between the edge-on and face-on orientation, depending on the choice of the solvent. The former orientation is realized in non-polar solvents and results in the formation of 1D π-stacks of TTF moieties that are held together by H-bonding of carboxyamide substituents. The latter orientation is enforced by the use of polar H-bonding solvents (alkanoic acids) which break intermolecular H-bonding and maximize the interaction of TTF molecules with the surface. In both cases, the surface density of TTFs is precisely defined by the length of the alkyl chain. Using Scanning Tunneling Microscopy and Atomic Force Microscopy, we show how the supramolecular assemblies observed at the liquid–solid interface can be transferred to growing dry films, thus paving the way for the application of such periodically structured materials in devices.
- This article is part of the themed collection: Celebrating 50 years of Professor Fred Wudl’s contributions to the field of organic semiconductors


 Please wait while we load your content...
Please wait while we load your content...