3,4-Phenylenedioxythiophenes (PheDOTs) functionalized with electron-withdrawing groups and their analogs for organic electronics†‡
Abstract
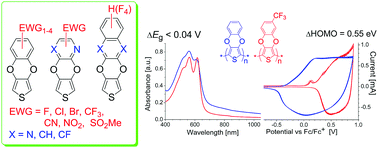
A novel, facile and efficient one-pot, microwave-assisted method of synthesis allowing an access to a new series of 3,4-phenylenedioxythiophene derivatives with electron-withdrawing groups at the benzene ring (EWG-PheDOT) and their analogs (with an expanded side π-system or with heteroaromatic rings, ArDOT) by the reaction of 2,5-dialkoxycarbonyl-3,4-dihydroxythiophenes with electrophilic aromatic/heteroaromatic compounds in dipolar aprotic solvents has been described. Its applicability over a wide range of novel functionalized ArDOTs as promising building blocks for organic electronic materials has been demonstrated. The structures of selected ArDOTs have been determined by single-crystal X-ray diffraction. The electronic structure of conjugated polymers p[ArDOTs] based on synthesized novel thiophene monomers has been studied theoretically by the DFT PBC/B3LYP/6-31G(d) method. The performed calculations reveal that while the side functional groups are formally not in conjugation with the polymer main chain, they have an unprecedentedly strong effect on the HOMO/LUMO energy levels of conjugated polymers, allowing their efficient tuning by over the range of 1.6 eV. In contrast to that, the energy gaps of the polymers are almost unaffected by such functionalizations and vary within a range of only ≤0.05 eV. Computational predictions have been successfully confirmed in experiments: cyclic voltammetry shows a strong anodic shift of p-doping for the electron-withdrawing CF3 group functionalized polymer p[4CF3-PheDOT] relative to the unsubstituted p[PheDOT] polymer (by 0.55 V; DFT predicted the decrease of the HOMO by 0.58 eV), while very similar Vis-NIR absorption spectra for both polymers in the undoped state indicate that their optical energy gaps nearly coincide (ΔEg < 0.04 eV).
- This article is part of the themed collection: Celebrating 50 years of Professor Fred Wudl’s contributions to the field of organic semiconductors


 Please wait while we load your content...
Please wait while we load your content...