A Friedländer route to 5,7-diazapentacenes†
Abstract
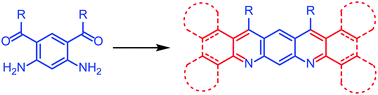
A route to compounds with a 5,7-diazapentacene skeleton has been established involving a Friedländer reaction. A diaminodiketone 8 has been made by a novel method and reacted with cyclohexanone to prepare an octa-hydro-5,7-diazapentacene 7a and with tetralone to produce a dibenzotetrahydro-5,7-diazapentacene 7b. Reaction of the diaminodiketone with a diarylethanone followed by oxidation gave a tetrabenzo-5,7-diazapentacene 18b. Compound 7b undergoes solid-state dimerization during single-crystal X-ray analysis. These materials possess lower frontier orbitals than pentacene and show strong absorption and fluorescence which is affected by the presence of acid. In particular 18b shows a remarkable colour change in solution upon addition of acid. These results suggest that suitably functionalised 5,7-diazapentacenes could be promising candidates for optoelectronic applications.
- This article is part of the themed collection: Celebrating 50 years of Professor Fred Wudl’s contributions to the field of organic semiconductors


 Please wait while we load your content...
Please wait while we load your content...