A new rigid diindolocarbazole donor moiety for high quantum efficiency thermally activated delayed fluorescence emitter†
Abstract
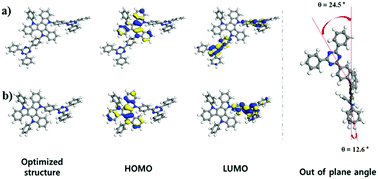
In this communication, we report an excellent new highly conjugated and rigid electron donor (diindolocarbazole) for thermally activated delayed fluorescence (TADF) emitters. The donor–acceptor structure with diindolocarbazole donor induces high oscillator strength as well as high probability for the radiative transition, suitable frontier molecular overlap with proper intramolecular charge transfer and a large dihedral angle with out-of-plane structure between electron donor and acceptor; this results in high photoluminescence quantum yield, small singlet–triplet energy splitting, and extremely short delayed fluorescence exciton lifetime. The fabricated organic light emitting diode with new donor applied green emitter exhibits a remarkably high external quantum efficiency of 31.4% without any out-coupling technique. It also shows extremely low efficiency roll-off characteristics with the highest luminance of 71 160 cd m−2 as well as good operational device stability.


 Please wait while we load your content...
Please wait while we load your content...