A supramolecular approach to construct a hydrolase mimic with photo-switchable catalytic activity†
Abstract
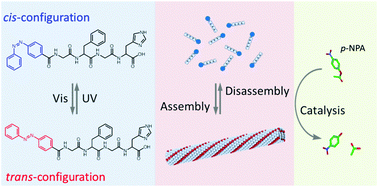
In this study, a peptide-based artificial hydrolase with photo-switchable activity was fabricated by the introduction of a catalytic histidine residue and a photo-responsive azobenzene group into the peptide chain. The peptide exhibits a structural transition from an antiparallel β-sheet to a random coil upon photo-irradiation, leading to the assembly/disassembly of the peptide fibril. An enhanced catalytic activity on p-nitrophenyl acetate was observed due to the proximity effect of the histidine residues and the hydrophobic microenvironment in the supramolecular assemblies. Under UV irradiation, this supramolecular system was destroyed together with a decline in the catalytic activity. Based on these photo-responsive properties, the activity of the hydrolase mimic can be reversibly controlled using UV and visible light. This study provides a new approach for constructing a switchable artificial enzyme based on a peptide material platform.


 Please wait while we load your content...
Please wait while we load your content...