Hierarchical theranostic nanomedicine: MRI contrast agents as a physical vehicle anchor for high drug loading and triggered on-demand delivery†
Abstract
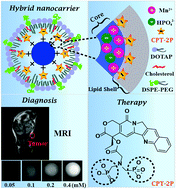
The loading of drugs and imaging agents in theranostic nanomedicines by a physical approach is usually poor (<5%), which limits their therapeutic effect and translation potential. We report a hierachical hybrid nanocarrier made of a manganese phosphate core, an outermost lipid shell, and an electrostatically deposited campothecin phosphonate interfacial middle layer. The hydrophobic interactions between camptothecin and lipid layers maintained the integrity and stability of the hybrid nanocarrier. Such a nanoplatform could surprisingly load camptothecin over 15% (w/w) with decent serum stability. The nanocarrier displayed pH-dependent cargo release profiles due to particle collapse under acidic conditions under which the r1 relaxivity of magnetic resonance imaging (MRI) was 25.2 mM−1 s−1 (pH 5.0). The nanocarrier could efficiently transport camptothecin into 4T1 cells with a half maximal inhibitory concentration of 5.4 ± 0.3 μM. Both in vivo MRI and fluorescence imaging analysis revealed that the nanocarrier could competently deliver the cargo to the tumor site. The anticancer efficacy of camptothecin-loaded nanocarrier was proved using the same 4T1 tumor-bearing mice model coupled with the histological and apoptosis analysis. This work not only presented a novel drug encapsulation approach, but also provided a new theranostic hybrid nanoplatform which could realize MRI-guided delivery of hydrophobic agents.


 Please wait while we load your content...
Please wait while we load your content...