Additives influence the phase behavior of calcium carbonate solution by a cooperative ion-association process†
Abstract
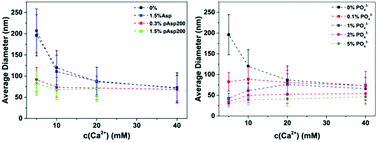
Amorphous calcium carbonate (ACC) has been widely found in biomineralization, both as a transient precursor and a stable phase, but how organisms accurately control its formation and crystallization pathway remains unclear. Here, we aim to illuminate the role of biologically relevant additives on the phase behaviour of calcium carbonate solution by investigating their effects on the formation of ACC. Results show that divalent cations like magnesium (Mg2+) ions and negatively charged small organic molecules like aspartic acid (Asp) have little/no effect on ACC formation. However, the particle size of ACC is significantly reduced by poly(aspartic acid) (pAsp) with long chain-length, but no effect on the position of the phase boundary for ACC formation was observed. Phosphate (PO43−) ions are even more effective in reducing ACC particle size, and shift the phase boundary for ACC formation to lower concentrations. These phenomena can be explained by a cooperative ion-association process where the formation of ACC is only influenced by additives that are able to attract either Ca2+ ions or CO32− ions and, more importantly, introduce an additional long range interaction between the CaCO03 complexes and promote the phase separation process. The findings corroborate with our proposed model of ACC formation via spinodal decomposition and provide a more realistic representation of how biology can direct mineralization processes.


 Please wait while we load your content...
Please wait while we load your content...