A bifunctional catalyst for efficient dehydrogenation and electro-oxidation of hydrazine†
Abstract
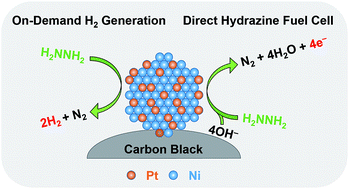
The chemical energy stored in energetic materials may often be utilized in various ways, which motivates the development of multifunctional catalysts for flexible and efficient utilization of the chemical energy. Hydrazine is a promising energy carrier due to its high energy density and high hydrogen content, which can be utilized as a chemical hydrogen storage medium or a fuel for direct fuel cells. Herein, we propose a bifunctional catalyst for efficient dehydrogenation and electro-oxidation of hydrazine. As a proof-of-concept study, a carbon-black-supported Pt0.2Ni0.8 nanoparticle catalyst has been developed with high activity and durability for both complete dehydrogenation (with a turnover frequency of 673 h−1 and a H2 generation rate of 188 L h−1 gmetal−1) and electro-oxidation (with a mass activity of 132 mA mgmetal−1) of hydrazine under mild conditions, outperforming other catalysts including Pt, Ni, Pd0.2Ni0.8, and Au0.2Ni0.8 nanoparticles. Such a bifunctional catalyst can enable the utilization of hydrazine as a promising energy carrier for both on-demand hydrogen generation and electricity generation via direct hydrazine fuel cells, enhancing its flexibility for future onboard applications.


 Please wait while we load your content...
Please wait while we load your content...