Dual carbon-protected metal sulfides and their application to sodium-ion battery anodes†
Abstract
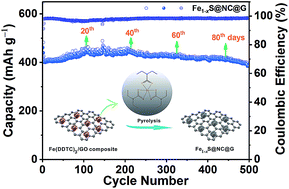
Metal sulfides are considered as promising anode materials for sodium ion batteries owing to their good redox reversibility and relatively high theoretical capacity. However, their cycle life and rate capability are still unsatisfactory because of poor conductivity and a large volume change during the discharge/charge processes. A facile method for preparing dual carbon-protected metal sulfides is reported. Metal diethyldithiocarbamate complexes are used as precursors. The synthesis only involves a co-precipitation of metal diethyldithiocarbamate complexes with graphene oxide and a subsequent thermal pyrolysis. As an example, N-doped carbon-coated iron sulfides wrapped in the graphene sheets (Fe1−xS@NC@G) are prepared and used as the anode material for a sodium ion battery. The as-synthesized Fe1−xS@NC@G electrode exhibits a high reversible capacity (440 mA h g−1 at 0.05 A g−1), outstanding cycling stability (95.8% capacity retention after 500 cycles at 0.2 A g−1), and good rate capability (243 mA h g−1 at 10 A g−1). Coupled with a Na3V2(PO4)2@C cathode, the full battery exhibits a high capacity retention ratio of 96.5% after 100 cycles and an average output voltage of ca. 2.2 V. More importantly, the proposed synthesis route is universal and can be extended to fabricate diverse transition metal sulfide-based composites with a dual carbon-protected nanostructure for advanced alkali ion batteries.


 Please wait while we load your content...
Please wait while we load your content...