Exploring polycyclic aromatic hydrocarbons as an anolyte for nonaqueous redox flow batteries†
Abstract
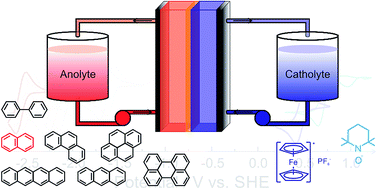
Nonaqueous redox flow batteries (RFB) can potentially achieve high energy density due to the extended operating voltage windows and redox-active material candidates. However, the development of reversible anode materials with a low redox potential and high solubility is still one of the main challenges. Here, we systematically explore polycyclic aromatic hydrocarbons (PAHs) and their corresponding radical anions (PAH˙n−) as anode redox-active couples with a combination of experimental and computational methods. The results reveal that naphthalene and its radical anion (Nap/Nap˙−) are a promising anode redox-active couple. Paired with catholytes separately containing ferrocenium hexafluorophosphate (FcPF6) and TEMPO, the resulting RFBs can provide theoretical maximum energy densities of 39 W h L−1 and 208 W h L−1, respectively, which are much higher than that of a traditional all-vanadium flow battery (25 W h L−1). As proofs of concept, both static-mode and flow-mode of the as-proposed RFBs are assembled and can deliver dozens of consecutive charge–discharge cycles.
- This article is part of the themed collection: 2018 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...