Directed self-assembly pathways of three-dimensional Pt/Pd nanocrystal superlattice electrocatalysts for enhanced methanol oxidation reaction†
Abstract
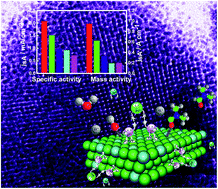
Although considerable interest has been devoted to revealing the formation of vigorous particles, very little is known about the regulating mechanism of these particles being self-assembled into higher-order supercrystals. Herein, we present a robust one-step approach to create three-dimensional (3D) Pt/Pd nanocrystal superlattices (NSLs) via self-assembly of Pt/Pd seeds anchored on polyvinylpyrrolidone chains into 3D networks with the assistance of Pt2+/Pd2+ coordinated N,N-dimethylformamide (DMF) as a bridging backbone linker. The 3D Pt/Pd NSLs are composed of well-defined interior Pt/Pd nanocrystals in a 3D face-centered cubic (fcc) superlattice oriented along the (111), (200) and (220) planes. EXAFS and XPS measurements confirm the strong synergy effect of Pt/Pd in 3D Pt/Pd NSLs due to the rich Pt0 species on the surface, predicting the enhanced electrocatalytic performance. The DFT calculations further reveal that the electrocatalytic enhancement of 3D Pt/Pd NSLs arises from the improved capability of methanol adsorption on metallic Pt active sites enriched by appropriate Pd alloying and surface nitrogen doping during the synthesis. The 3D Pt/Pd NSLs show a high current density of 952 mA mg−1, 4.7 times higher than that of the commercial Pt/C catalyst. The self-assembly pathways may be extended to synthesize other 3D noble metal NSLs with enhanced electrocatalytic performance for direct methanol fuel cells.


 Please wait while we load your content...
Please wait while we load your content...