The progress of metal-free catalysts for the oxygen reduction reaction based on theoretical simulations
Abstract
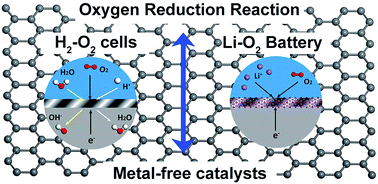
As a major determining factor on the performance of fuel cells and metal–air batteries, the cathodic oxygen reduction reaction (ORR) plays a vital role in electricity production. The extremely complicated reaction process during ORR catalysis makes theoretical simulations highly important to clarify its catalytic mechanism as well as to develop high-performance catalysts. To substitute high-cost and scarce noble-metal catalysts (like Pt-based catalysts), novel metal-free ORR catalysts have been widely proposed by experimental and theoretical approaches. In this review, we summarize recent theoretical progress on the microscopic reaction process and catalytic performance of metal-free ORR catalysts, in both fuel cells and metal–air batteries (mainly Li–O2 batteries). The metal-free ORR catalysts are sorted by the origin of catalytic activity, such as defects and doping, intrinsic hybridization, substrate interaction, as well as alloying. Based on the presented research results, we summarize the theoretical simulations on revealing the ORR process catalyzed by metal-free catalysts, which is helpful for designing high-performance metal-free catalysts to substitute the traditional noble-metal catalysts.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...