Electrolyte-assisted hydrothermal synthesis of holey graphene films for all-solid-state supercapacitors†
Abstract
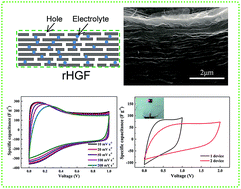
A novel strategy was presented for the synthesis of graphene-based film electrodes by electrolyte-induced hydrothermal reduction of holey graphene oxide based films. After hydrothermal treatment, the reduced holey graphene films (rHGFs) maintained their flexibility, integrity and porosity, which resulted from the electrolyte-induced balance between electrostatic repulsion and π–π attraction. The trapped electrolyte (H2SO4) between graphene layers can prevent aggregation, and the resulting supercapacitor exhibits a high specific capacitance in the sulfuric acid electrolyte. Benefitting from the in-plane pores and oxygen-containing groups on graphene sheets, the rHGF electrode exhibits a high specific capacitance of 260 F g−1 (297 F cm−3) with remarkable rate performance in a three-electrode system. To evaluate the practical application, flexible all-solid-state supercapacitors based on the rHGF electrode with PVA/H2SO4 gel electrolyte were prepared, and the device exhibits remarkable cycling stability. The areal capacitance and volumetric energy density were 56 mF cm−2 and 1.41 W h cm−3, respectively. This work demonstrated a cost-effective and simple technique to prepare compact graphene films with continuous ion channels and showed great importance on the design of flexible, portable and highly integrated supercapacitors.


 Please wait while we load your content...
Please wait while we load your content...