Stannate derived bimetallic nanoparticles for electrocatalytic CO2 reduction†
Abstract
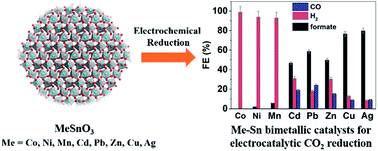
The synthesis of 8 metal–Sn (metal = Mn, Co, Ni, Cu, Zn, Ag, Cd, and Pb) bimetallic materials by electrochemical reduction of their metal stannates is reported. When the metal–Sn bimetallic materials were used as electrocatalysts for electrochemical CO2 reduction, bulk electrolysis results revealed that the Ag–Sn and Cu–Sn bimetallic systems showed the highest activity and selectivity for formate. When incorporated with reduced graphene oxide (rGO), their electrocatalytic performance can be further improved, making them among the best performing tin based CO2 reduction electrocatalysts reported so far. The Ag–Sn/rGO catalyst reaches the highest faradaic efficiency for formate (FEformate) of 88.3% at −0.94 V vs. RHE with a current density of 21.3 mA cm−2 in a 0.5 M aqueous NaHCO3 solution. Comparable performance was observed from the Cu–Sn/rGO catalyst, where the highest FEformate of 87.4% and a current density of 23.6 mA cm−2 were obtained at −0.99 V vs. RHE. Both catalysts exhibited high stability over a 6 hour electrolysis period with the FEformate variation being less than 2%. The excellent performance of this class of bimetallic nanoparticle/rGO composite catalysts is attributed to the small size of the stannate derived bimetallic nanoparticles, the presence of a SnOx layer and the introduction of rGO which prevents the aggregation of the bimetallic nanoparticles and provides a 3D conductive network to facilitate fast charge transfer. This study demonstrates a facile yet general strategy that enables the synthesis of bimetallic systems for highly efficient electrocatalytic CO2 reduction.


 Please wait while we load your content...
Please wait while we load your content...
