A facile access to stiff epoxy vitrimers with excellent mechanical properties via siloxane equilibration†
Abstract
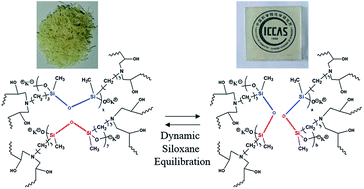
A new high performance stiff epoxy vitrimer based on siloxane equilibration has been fabricated in a simple way in which a polysiloxane oligomer containing aminopropyl side groups and catalytic potassium silanolate end groups was synthesized and used as a curing agent. The viscoelastic properties of the cured epoxy can be controlled by varying the concentration of potassium silanolate groups and the vitrimer possessing a complete stress relaxation behavior can be prepared, with relaxation times ranging from 376.8 s at 90 °C to 40.2 s at 170 °C. Due to the dynamic siloxane equilibration of polysiloxane catalyzed by potassium silanolate groups, this epoxy vitrimer exhibits excellent self-healing and recycling abilities. It can self-repair with a high healing efficiency, and be recycled at 130 °C within 40 min, retaining its original mechanical and thermal properties after at least four recycling cycles. Furthermore, such a material exhibits simultaneously a high service temperature (a glass transition temperature of 83 °C and an initial degradation temperature of 358 °C) and a strong (a stress at break of 46.6 MPa) and stiff (Young's modulus of 2.2 GPa) nature.


 Please wait while we load your content...
Please wait while we load your content...