Co-mixing hydrogen and methane may double the energy storage capacity†
Abstract
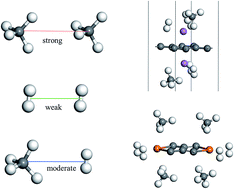
The use of hydrogen fuel as clean energy is hindered by the low capacity of the storage medium. Although the combustion energy of a CH4 molecule is three times higher than that of H2, the same medium can adsorb much fewer CH4 molecules than H2 due to the much stronger inter-molecular repulsion of the former. Here, we show, from first-principles theoretical calculations, that mixing hydrogen and methane gas may significantly increase the energy storage capacity compared with either pure hydrogen or methane. The repulsion between hydrogen and methane molecules is moderate and the open metal sites on a surface can be effectively used to increase the energy storage capacity. Using two different surfaces (graphene and graphene nanoribbons) decorated with alkali or 3d transition metal atoms, as examples, we show that the energy storage capacity can be approximately doubled by this mixing and an equivalent gravimetric hydrogen density of 14.0 wt% can be obtained. This approach can be applied to most current storage media with open metal sites.


 Please wait while we load your content...
Please wait while we load your content...
