An in situ approach for the synthesis of a gum ghatti-g-interpenetrating terpolymer network hydrogel for the high-performance adsorption mechanism evaluation of Cd(ii), Pb(ii), Bi(iii) and Sb(iii)†
Abstract
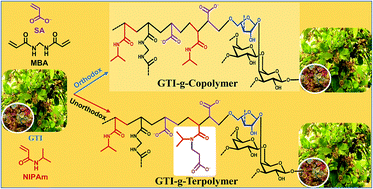
Herein, gum ghatti-g-(N-isopropylacrylamide-co-3-(N-isopropylacrylamido) propanoic acid-co-sodium acrylate) (GTINIAMSA), a novel interpenetrating terpolymer network hydrogel with unprecedented thermomechanical and physicochemical properties and excellent recyclability, was synthesized via the grafting of gum ghatti (GTI) and the in situ strategic protrusion of 3-(N-isopropylacrylamido) propanoic acid (NIPAMPA) during the solution polymerization of sodium acrylate (SA) and N-isopropylacrylamide (NIPAm) using N,N′-methylenebisacrylamide (MBA) and potassium persulfate (PPS)/sodium bisulfite (SBS) as a cross-linker and redox pair of initiators, respectively, through the systematic multistage optimization of the constituents and the reaction temperature for a comprehensive understanding of the superadsorption mechanism of hazardous metal ions, i.e. M(II/III), like Cd(II), Pb(II), Bi(III) and Sb(III). The unorthodox in situ attachment of NIPAMPA, grafting of GTI into NIPAm-co-NIPAMPA-co-SA (NIAMSA) matrix and the superadsorption mechanism were systematically investigated via extensive unloaded and/or loaded microstructural analyses by FTIR, 1H-/13C-NMR, C 1s-/O 1s-/Cd 3d5/2,3/2-/Pb 4f7/2,5/2-/Bi 4f7/2,5/2-/Sb 3d3/2-XPS, TGA, XRD, FESEM and EDX, along with the measurements of the %gel content (% GC), pH at point of zero charge (pHPZC), % graft ratio (% GR) and network parameters of the hydrogels. The prevalence of ionic (I) and variegated coordinative interactions, such as monodentate (M), bidentate bridging (BB) and bidentate chelation (BC), between GTINIAMSA and M(II/III) were also investigated by FTIR and the fitting of kinetics data to a pseudo-second-order model as well as by the measurements of the activation energies of adsorption. The equilibrium adsorption data of M(II/III) were fitted excellently to the Langmuir isotherm model. The thermodynamically spontaneous chemisorption processes showed the excellent maximum adsorption capacities (ACs) of 1477.83, 1568.81, 1582.38 and 1518.09 mg g−1 for Cd(II), Pb(II), Bi(III), and Sb(III), respectively, at 303 K with an adsorbent dose of 0.02 g and initial concentration of M(II/III) = 500–800 ppm.


 Please wait while we load your content...
Please wait while we load your content...