Unravelling the reaction chemistry and degradation mechanism in aqueous Zn/MnO2 rechargeable batteries†
Abstract
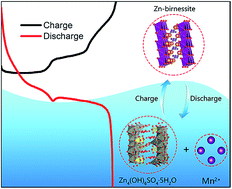
Aqueous Zn/MnO2 rechargeable batteries utilizing a near neutral electrolyte have demonstrated great potential for large-scale energy storage applications, due to their safe and sustainable nature. Nevertheless, the reaction chemistry and degradation process associated with the MnO2-based cathode is not yet fully understood. Herein, a novel reversible Zn/MnO2 battery with zinc hydroxide sulfate (Zn4(OH)6SO4·5H2O, ZHS) as the cathode has been designed, where active MnO2 is formed in situ during the initial charge process from the Mn(II)-containing ZnSO4 electrolyte. A combination of electrochemical and material characterizations reveal two-step redox reactions (Mn(II) ions ⇌ ZnMn2O4 spinel ⇌ layered Zn-birnessite) during the charge–discharge process. Excellent cycling stability with a capacity retention of 100% after 1500 cycles is achieved at 500 mA g−1. The mechanism for long-term capacity fading is also studied. Cycling reversibility is destroyed by the irreversible consumption of Mn(II) to form woodruffite with a tunnel structure and poor electrochemical activity.


 Please wait while we load your content...
Please wait while we load your content...