One-step growth of nitrogen-decorated iron–nickel sulfide nanosheets for the oxygen evolution reaction†
Abstract
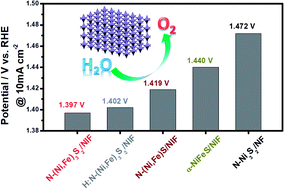
The large overpotential loss of the oxygen evolution reaction (OER) is a major obstacle restricting the wide commercial application of water-splitting devices. Herein, we report one-step growth of nitrogen-decorated iron–nickel sulfide nanosheet arrays on a conductive Ni–Fe alloy foam (N–(Ni,Fe)3S2/NIF). It is noteworthy that N–(Ni,Fe)3S2/NIF has an ultra-low overpotential of 167 mV at 10 mA cm−2 and exhibits the best OER performance among the non-precious electrocatalysts reported so far. Furthermore, N–(Ni,Fe)3S2/NIF shows nearly no degradation after a long-term OER test for 50 h at a constant current density of 10 mA cm−2. The superior catalytic activity and stability for the OER is due to the application of simultaneous regulation of specific morphologies, incorporation of Fe, and surface nitrogen-anion decoration on Ni3S2 materials. In addition, N–(Ni,Fe)3S2/NIF prepared by another method also shows excellent OER activity. This work will pave a new way to develop advanced electrocatalysts with a lower OER overpotential.


 Please wait while we load your content...
Please wait while we load your content...