Ionic liquids as an efficient medium for the mechanochemical synthesis of α-AlH3 nano-composites†
Abstract
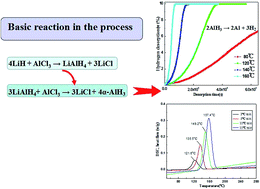
Aluminum hydride (AlH3) is one of the most promising hydrogen storage materials that has a high theoretical hydrogen storage capacity (10.08 wt%) and relatively low dehydriding temperature (100–200 °C). In this work, we present a cost-effective route to synthesize the α-AlH3 nano-composite by using cheap metal hydrides and aluminum chloride as starting reagents and to achieve liquid state reactive milling. The LiH/AlCl3 and MgH2/AlCl3 reaction systems were systemically explored. The phase identification of the obtained products was carried out by XRD and the morphology observed by TEM characterization. It was found that the α-AlH3 nano-composite can be successfully synthesized by reactive milling of commercial AlCl3 and LiH in a neutral ionic liquid ([2-Eim] OAc). Based on XRD analysis and TEM observation, an average grain size of 56 nm can be obtained by the proposed mechanochemical process. By setting the isothermal dehydrogenation temperature between 80 and 160 °C, the as-synthesized α-AlH3 nano-composite exhibits an advantage in hydrogen desorption capacity and has fast dehydriding kinetics. The hydrogen desorption content of 9.93 wt% was achieved at 160 °C, which indicates the potential utilization of the prepared nanocomposite in hydrogen storage applications.


 Please wait while we load your content...
Please wait while we load your content...