Abstract
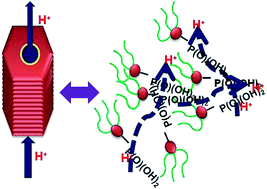
In polymer based ionic conductors, the conductivity is suppressed by a low degree of chain mobility, therefore it is imperative to design a molecular system in which ionic groups can be mobilized and immobilized as a function of temperature to allow ions to move freely as well as in a controlled manner with a low energy barrier. Herein, we report an innovative approach to combine both the concepts of self-assembly and disassembly of the functionalized molecules to investigate anhydrous ionic (proton) conduction and related activation energy (Ea). For this purpose, organic proton conductors are designed in such a way that self-assembly of the molecules can occur via non-covalent interactions giving rise to an organized solid state in which ionic groups are held together via a network of hydrogen bonds. A new class of anhydrous ionic conductors with hydrophobic and hydrophilic counterparts namely alkyl chains and a phosphonic acid group, respectively, are investigated for fuel cell applications. The highest anhydrous proton conductivity of up to 10−2 S cm−1 at 140 °C is recorded for these ionic molecules. Thermal gravimetric analysis of these materials demonstrates their stability up to 190 °C and thereby their ability to perform at high temperature.


 Please wait while we load your content...
Please wait while we load your content...