Investigation of the exceptional charge performance of the 0.93Li4−xMn2O5–0.07Li2O composite cathode for Li-ion batteries†
Abstract
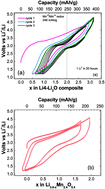
Herein, we report a detailed study on the high-energy density nanostructured Li4−xMn2O5–Li2O composite with a high discharge capacity of 355 mA h g−1, constituting the highest value reported to date for a lithium–manganese oxide electrode. Its high capacity was previously wrongly attributed to the participation of Li4−xMn2O5 (Li4) alone. However, more detailed compositional, structural, and electrochemical analyses revealed the important role played by Li2O during battery cycling that was able to increase the original capacity of Li4 by 100 mA h g−1. The participation of Li2O is evident during the first charge when it is mixed on the nanoscale with lithiated manganese oxide. The fully delithiated phase Li0 is formed after the first charge. Our structural study combining neutron and synchrotron diffraction and transmission electron microscopy demonstrates that lithium exchange essentially implies breathing of the average cubic framework accompanied by small local atomic rearrangements that mainly involve oxygen anions.


 Please wait while we load your content...
Please wait while we load your content...