Electrochemical reduction of CO2 on defect-rich Bi derived from Bi2S3 with enhanced formate selectivity†
Abstract
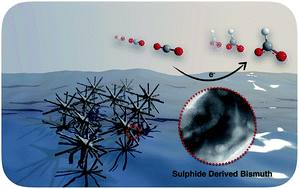
A sulphide-derived bismuth catalyst, synthesised from a one-pot hydrothermal reaction followed by electrochemical reduction, exhibits excellent performance for converting CO2 into formate in an aqueous bicarbonate medium with high activity, selectivity and durability. The maximum faradaic efficiency of 84.0% for formate formation was achieved at an overpotential of 670 mV. A detailed study reveals that the lattice defects associated with the sulphide-derived Bi rather than residual sulphur are likely to engender a positive effect on the catalytic reduction of CO2.


 Please wait while we load your content...
Please wait while we load your content...