The synergistic effect of cobalt oxide and Gd-CeO2 dual infiltration in LSCF/CGO cathodes†
Abstract
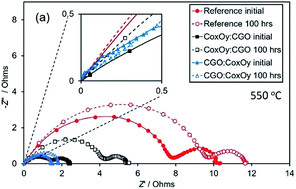
La0.6Sr0.4Co0.2Fe0.8O3−δ/Ce0.9Gd0.1O1.9 composite cathodes were nano-engineered via “dual” inkjet printing infiltration of nitrate salt solutions in a single step procedure. After calcination in air at 700 °C the cathodes were decorated with Ce0.9Gd0.1O1.9 and CoxOy nanoparticles (∼20 nm in size). The effects of the as-created nano-decoration on the electrochemical activity and the performance stability in the intermediate temperature range (500–700 °C) were investigated. The nano-engineered microstructure was found to extend the active three-phase boundary and to promote adsorption–dissociation–surface exchange reactions. Electrochemical impedance tests conducted on symmetric cells showed a reduction in the polarisation resistance of between 1.5 and 7.0 times depending on temperature (500–700 °C). High-resolution X-ray photoelectron spectroscopy and in situ high temperature Raman spectroscopy were used to study aging and thermal cycling effects on the cathodes' surface chemistry. Aging tests of the infiltrated electrodes up to 100 hours in air revealed an enhanced stability of the decorated electrodes ascribed to the suppression of SrO surface segregation. This work demonstrated that the sequence of infiltration of both inks introduces noticeable differences in the oxygen reduction reaction.


 Please wait while we load your content...
Please wait while we load your content...