Improved capacity of redox-active functional carbon cathodes by dimension reduction for hybrid supercapacitors†
Abstract
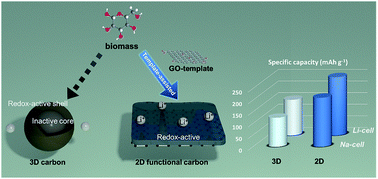
Hybrid supercapacitors, which combine the advantages of supercapacitors and rechargeable batteries, have the potential to meet the demands of both high-energy and -power in electrochemical energy storage systems. However, the energy density of the hybrid supercapacitors has been limited because of the low capacity of the activated carbon cathode. Here we introduce a high-capacity carbon cathode containing plenty of oxygen functional groups that are redox-active towards both Li- and Na-ions. This functional carbon has an ultra-thin two-dimensional structure that has significant advantages in utilizing the redox reactions. The functional carbon cathode can exhibit very high capacities of ∼250 mA h g−1 in Li-cells and ∼210 mA h g−1 in Na-cells. A hybrid supercapacitor consisting of the two-dimensional functional carbon cathode with a commercial level loading density of ∼9.3 mg cm−2 and a Si-based anode delivers a high-energy density of ∼182 W h kg−1 at a high-power density of 1 kW kg−1.


 Please wait while we load your content...
Please wait while we load your content...