The enhanced electrochemical response of Sr(Ti0.3Fe0.7Ru0.07)O3−δ anodes due to exsolved Ru–Fe nanoparticles
Abstract
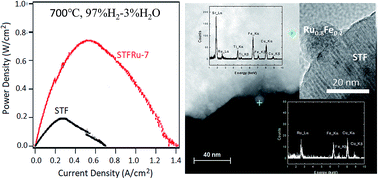
A mixed conducting oxide with a nominal composition Sr(Ti0.3Fe0.7Ru0.07)O3−δ (STFRu) is studied, in comparison with SrTi0.3Fe0.7O3−δ (STF) oxide, as an anode for solid oxide fuel cells. Exposing STFRu to reducing fuel conditions at 800 °C for 4 h results in the exsolution of essentially all of the Ru and a small fraction of the Fe from the oxide, and the formation of Ru1−xFex nanoparticles on the oxide surfaces. Most of the nanoparticles have the hexagonal structure expected for Ru-rich alloys, and thermogravimetric analysis suggests the composition x ∼ 0.2. A small fraction of bcc-structure, presumably Fe-rich, nanoparticles are also detected. Comparison of cells with STFRu and STF anodes shows that the presence of Ru induces a reduced polarization resistance and increases the maximum power density under most cell operating conditions, particularly at lower temperatures and hydrogen partial pressures. For example, at 700 °C and 30% H2 fuel, the maximum power density is 0.1 W cm−2 for STF compared to 0.3 W cm−2 for STFRu. There is also a significant change in the shape of the current–voltage curves and the pH2-dependence of the anode polarization resistances RP,A ∝ (pH2)−m, from m ∼ 0.5–1.0 for STF to m ∼ 0.11–0.29 for STFRu; these suggest that Ru1−xFex nanoparticles improve anode performance by promoting hydrogen adsorption.


 Please wait while we load your content...
Please wait while we load your content...