Tracking precursor degradation during the photo-induced formation of amorphous metal oxide films†
Abstract
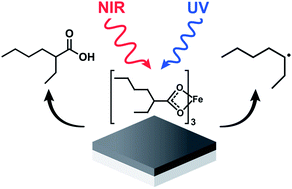
The formation of thin films of amorphous iron oxide (a-FeOx) produced by the ultraviolet (UV) or near infrared (NIR) driven decomposition of iron(III) tris-(2-ethylhexanoate) cast on a substrate was investigated. The gaseous byproducts liberated during each of the two distinctive photodecomposition reactions, denoted UV driven decomposition (UVDD) and NIR driven decomposition (NIRDD), were analyzed by gas chromatography mass spectrometry (GC/MS). The oxide films were analyzed by FTIR spectroscopy, grazing incidence X-ray diffraction (GIXRD) and electrochemical methods. These analyses collectively indicate that the UVDD pathway proceeds through a succession of reactions mediated by light-induced ligand-to-metal charge transfer (LMCT) processes that produce a heptyl radical byproduct and a reduced metal that is reactive towards ambient dioxygen. NIRDD is shown to follow thermally driven decomposition pathways that do not produce radicals. The oxide layers formed using both processes are nonetheless strikingly similar in structure and properties, including electrocatalytic activity towards the oxygen evolution reaction.


 Please wait while we load your content...
Please wait while we load your content...