Light-induced reactivity of gold and hybrid perovskite as a new possible degradation mechanism in perovskite solar cells†
Abstract
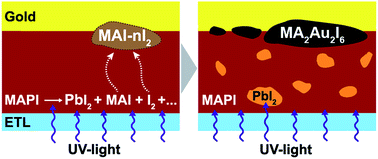
We suggest a new degradation mechanism of commonly used gold electrodes in hybrid perovskite solar cells (PSCs) originating from chemical interaction between gold and highly reactive iodine-containing byproducts formed in the course of perovskite decomposition. Intensive UV-irradiation of perovskite would typically lead to the release of volatile I2 and CH3NH3I (MAI) resulting in the formation of recently reported highly reactive polyiodide melts (RPMs) with the general formula MAI–nI2. These RPMs react aggressively with metallic gold at room temperature causing the formation of [AuI2]− and [AuI4]− complexes and, consequently, precipitation on the gold interface of a new (MA)2Au2I6 phase with a tetragonal symmetry. The high rate and depth of this reaction renders gold an easy target for attack under these particular conditions despite its notorious chemical inertness, thus making gold unsuitable for widespread use in iodine-based perovskite solar cells; other cheaper and more stable materials are needed as a better choice for further development in this area.


 Please wait while we load your content...
Please wait while we load your content...