Enhanced oxygen electroreduction over nitrogen-free carbon nanotube-supported CuFeO2 nanoparticles†
Abstract
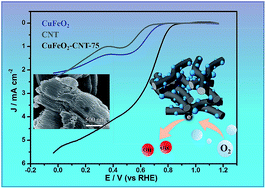
Composites of nano-sized delafossite CuFeO2 with 10–20 nm diameter and carbon nanotubes (CNTs) are constructed through a facile, one-pot hydrothermal process. The obtained nitrogen-free composites demonstrate promising electrocatalytic activity for the oxygen reduction reaction (ORR) in alkaline media. The introduction of CNTs facilitates the formation of nano-sized CuFeO2 particles. The synergistic effect between the nanosized delafossite CuFeO2 and carbon nanotubes endows the composites with a high electron transfer ability contributing to their robust ORR performance in 0.1 M KOH. This synthetic approach represents a promising method for the preparation of non-precious metal based electrocatalytic materials.


 Please wait while we load your content...
Please wait while we load your content...