Ultrahigh rate sodium ion storage with nitrogen-doped expanded graphite oxide in ether-based electrolyte†
Abstract
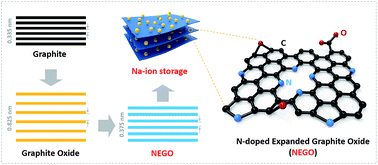
Exploring anode materials with excellent rate performance and high initial coulombic efficiency (ICE) is crucial for lithium/sodium-ion batteries (LIBs/SIBs). However, it is still very challenging to achieve this goal in a cost-effective way, particularly for SIBs. Herein, graphite oxide, was treated in ammonia atmosphere for a balance between the oxygen- and nitrogen-contained functional groups and yielded nitrogen-doped expanded graphite oxide (NEGO). Electrochemical characterizations were systematically carried out in ether and ester-based electrolytes to shed light on the storage mechanism of NEGO in SIBs. The ICE of NEGO employed in ether-based electrolyte improves to 72.08% from that in ester-based electrolyte (24.73%). Moreover, the as-synthesized NEGO exhibits ∼125 mA h g−1 and ∼110 mA h g−1 capacities in ether and ester-based electrolytes, respectively, even under a record high current density (30 A g−1). Expanded surface area and nitrogen doping significantly increase the active sites and decrease the electrical resistivity from 140 Ω (EGO) to 40 Ω (NEGO) by removing excess oxygen. Moreover, small amounts of residual oxygen, particularly quinone and carboxyl, along with nitrogen occupied sites offer additional pseudocapacitance. Considering the advantages in scale-up and cost-effective production, NEGO is a promising low-cost anode material for SIBs. This study also provides strategies for the design of electrolyte for SIBs to realize practical applications in power-grid energy storage.


 Please wait while we load your content...
Please wait while we load your content...