Comprehensive investigation of the lithium insertion mechanism of the Na2Ti6O13 anode material for Li-ion batteries†
Abstract
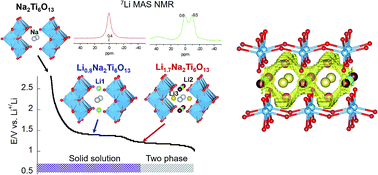
Sodium hexatitanate Na2Ti6O13 with a tunnel structure has been proposed to be an attractive anode material for lithium ion batteries because of its low insertion voltage, structural stability and good reversibility. In order to obtain a full understanding of the properties of this titanate, a combination of in situ synchrotron X-ray diffraction, neutron diffraction and 7Li MAS solid-state NMR spectroscopy is used in the present work. During the first insertion stage (centered at 1.3 V), lithium is allocated in square planar LiO4 2c (Li1) sites, minimizing electrostatic repulsion with Na ions. During the second lithium uptake (centered at 1.1 V), Li ions pass from 2c to empty 4i (Li2) sites of y/b = 0.5 planes, near Ti3+ cations. Distribution of Li+ and Na+ ions with respect to Ti3+ cations was deduced from Fourier map differences (Rietveld technique) and NMR quantitative analyses in Li0.8Na2Ti6O13 and Li1.7Na2Ti6O13 samples. 7Li MAS-NMR analysis showed that Li ions occupy three fourfold coordinated sites with reasonable Li+–O–Ti3+ bond distances, while Na cations remain at eightfold coordinated positions near Ti4+ cations as deduced from 23Na MAS-NMR spectroscopy. 7Li MAS-NMR recorded at increasing temperatures suggests that Li ions move along sinusoidal paths to reduce Li–Na electrostatic interactions. Li mobility along the b-axis is favored by partial occupation of interstitial 4i sites (Li3) located at both sides of Na cations in y/b = 0 planes. In lithium inserted samples the most probable – Li1(2c) → Li3(4i) → Li1(2c) – conduction paths were deduced. However, formation of Li pairs at y/b = 0 planes (Li2 sites), where Li ions are located near Ti3+ cations, reduces the amount of mobile Li ions that participate in conduction processes. Proximity of lithium to Li and Na ions limits insertion to ca. 2 Li ions per structural formula.


 Please wait while we load your content...
Please wait while we load your content...