Engineering the coordination geometry of metal–organic complex electrocatalysts for highly enhanced oxygen evolution reaction†
Abstract
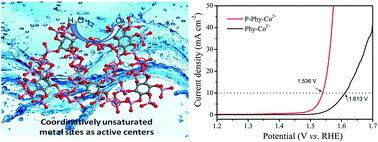
Designing highly efficient oxygen evolution reaction (OER) electrocatalysts is very important for various electrochemical devices. In this work, for the first time, we have successfully generated coordinatively unsaturated metal sites (CUMSs) in phytic acid–Co2+ (Phy–Co2+) based metal–organic complexes by engineering the coordination geometry with room-temperature plasma technology. The CUMSs can serve as active centers to catalyze the OER. The electron spin resonance and X-ray absorption spectra provide direct evidence that the coordination geometry is obviously modified with many CUMSs by the plasma treatment. The plasma treated Phy–Co2+ (P-Phy–Co2+) only requires an overpotential of 306 mV to reach 10 mA cm−2 on glassy carbon electrodes. When we expand this strategy to a CoFe bimetallic system, it only needs an overpotential of 265 mV to achieve 10 mA cm−2 with a small Tafel slope of 36.51 mV dec−1. P-Phy–Co2+ is superior to the state-of-the-art. Our findings not only provide alternative excellent OER electrocatalysts, but also introduce a promising principle to design advanced electrocatalysts by creating more CUMSs.


 Please wait while we load your content...
Please wait while we load your content...