Influence of annealing at intermediate temperature on oxygen transport kinetics of Pr2NiO4+δ
Abstract
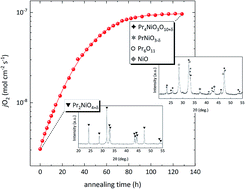
Electrical conductivity relaxation (ECR) and oxygen permeation measurements were conducted, at 750 °C, to assess the long-term oxygen transport characteristics of the mixed ionic–electronic conducting Pr2NiO4+δ with a K2NiF4 structure. The results show that the apparent values for the oxygen diffusion and surface exchange coefficients extracted from the data and the associated oxygen flux increase over 120 h by 1–2 orders of magnitude. The results of post-mortem X-ray diffraction analysis of the samples show partial to virtually complete decomposition of Pr2NiO4+δ under the conditions of the experiments to Pr4Ni3O10+δ, PrNiO3−δ, Pr6O11, and traces of NiO. Pulse 18O–16O isotopic exchange (PIE) measurements confirmed fast surface exchange kinetics of the higher-order Ruddlesden–Popper phase Pr4Ni3O10+δ and Pr6O11 formed upon decomposition. Additional factors related to the microstructure, however, need to be considered to explain the observations.


 Please wait while we load your content...
Please wait while we load your content...