Hollow POM@MOF hybrid-derived porous Co3O4/CoMoO4 nanocages for enhanced electrocatalytic water oxidation†‡
Abstract
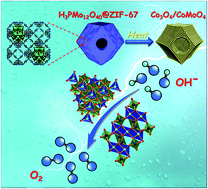
Tailoring highly active and robust electrocatalysts for the sluggish oxygen evolution reaction (OER) is a crucial key to electrochemical water splitting. Here, we report a facile in situ self-assembly strategy to encapsulate a strong Brønsted acid, H3PMo12O40, in the customized cavities of an acid-vulnerable Co-based metal–organic framework (ZIF-67), generating a series of unique hollow H3PMo12O40@ZIF-67 hybrids. The calcination of the hybrids in air leads to porous Co3O4/CoMoO4 nanocages consisting of ultrafine crystallites, which provide rich active sites for surface redox reactions. Compared with Co3O4 derived from pristine ZIF-67, the optimal Co3O4/CoMoO4-50 exhibits significantly enhanced OER catalytic activity with a low onset potential of 1.47 V (vs. the RHE) and an almost constant overpotential of 318 mV to maintain a stable current density of 10 mA cm−2 in alkaline media for at least 15 h. These electrochemical performances are superior to most of the MOF-derived OER catalysts and highly competitive among reported non-precious metal-based OER catalysts. Furthermore, our study confirms that the suitable concentration of Co2+ in the octahedral sites plays an important role in the enhanced OER activity of Co3O4/CoMoO4-50.


 Please wait while we load your content...
Please wait while we load your content...