Barium carbonate as a synergistic catalyst for the H2O/CO2 reduction reaction at Ni–yttria stabilized zirconia cathodes for solid oxide electrolysis cells†
Abstract
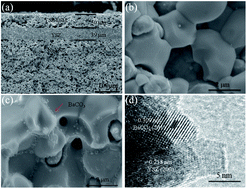
BaCO3 is primarily demonstrated as an excellent synergistic catalyst for efficient electro-reduction of H2O/CO2 to H2/CO at the state-of-the-art Ni–YSZ (yttria-stabilized zirconia) cathodes for solid oxide electrolysis cells (SOECs) with YSZ electrolytes and strontium doped lanthanum manganite oxygen electrodes. BaCO3 nanoparticles, which are deposited onto porous Ni–YSZ using the infiltration process, can significantly improve the SOEC performance such as increasing the electrode interfacial polarization conductivity over 50% and promoting the electrolysis current density by a factor of up to 2 for the electrolysis of H2O to H2, CO2 to CO, and co-electrolysis of H2O–CO2 to H2–CO at 800 °C. In addition, it seems that BaCO3 can improve the SOEC durability for the co-electrolysis of H2O–CO2. The improvement is associated with an enhanced surface charge transfer process, which is the rate-determining step for the reduction reaction, as shown by analyzing the AC impedance spectrum measured with a three-electrode configuration. The highest improvement is achieved with CO2 reduction possibly due to the additional enhancement in the adsorption/dissociation process by BaCO3 as revealed with temperature programmed CO2 desorption analysis.


 Please wait while we load your content...
Please wait while we load your content...